Written by Robert Whitaker / Mad in America
When Mad in America received a notice this past June that Joanna Moncrieff, Mark Horowitz, and colleagues would soon publish a paper concluding that there were no research findings that supported the low-serotonin hypothesis of depression, I initially wondered whether we should bother to report on it. Mad in America readers know well that the low-serotonin theory had long ago been debunked, with numerous articles on our site telling of that fact, and so I quipped to other MIA staff that reviewing the article would be like “beating a dead horse.”
But such is our little cocoon here at Mad in America. For much of the mainstream media, their paper made for a stunning finding. In print, radio, and television, the paper has been described as a “landmark” finding, as a “game changer” and so forth, the media telling of how it has shaken up accepted wisdom about antidepressants and “how they work.”
This was rather amusing, I thought, as the exclamations of surprise revealed the media’s utter failure regarding their reporting on psychiatry for the past decades. Their surprise served as a tacit confession that they had been publishing propaganda for some time.
Then, as psychiatrists publicly commented on the paper, a second confession appeared, this one indeed of “landmark” importance. Their comments serve as an admission that, for the past several decades, their profession committed medical fraud. And I am using that term in its legal sense.
As Moncrieff and colleagues noted, there is a long line of research that failed to find evidence supporting the low-serotonin theory of depression. What was new about their work was that they performed a comprehensive review of this research, looking at the different “types” of studies that had been done, and finding that all had failed to produce evidence supporting the theory. In response, a number of prominent psychiatrists in the UK and the United States dismissed the paper as old news. Here is a sampling:
From UK psychiatrists:
“The findings from this review are really unsurprising. Depression has lots of different symptoms and I don’t think I’ve met any serious scientists or psychiatrists who think that all causes of depression are caused by a simple chemical imbalance in serotonin.” —Michael Bloomfield, University College London (UCL)
“This paper does not present any new findings but just reports results which have been published elsewhere and it is certainly not news that depression is not caused by ‘low serotonin levels.’” —David Curtis, UCL Genetics Institute
From US psychiatrists:
“Nothing is new here. And the fuss surrounding the paper reveals much ignorance about psychiatry. The serotonin hypothesis of depression which became popular from the 1990s until now, is false, and has known to be false for a long time, and never was proven to begin with.” —Nassir Ghaemi, Tufts University School of Medicine
“When I was doing research for [my] book, I was reading the same studies that I am sure that Dr. Moncrieff and colleagues read, which were basically saying that there’s no direct evidence of a serotonin deficiency. So it’s not really new.” —Daniel Carlat, publisher of the Carlat Psychiatry Report
The psychiatrists making these comments are correct. The psychiatric research community has long known that the low-serotonin theory didn’t pan out and that, in fact, the field long ago moved on to new theories about the possible pathology that gives rise to depression. Yet, as is easy to show, the American Psychiatric Association, in concert with pharmaceutical companies, promoted the low-serotonin theory to the public long after the low-serotonin theory had been found to be without merit. Scientific advisory councils populated by professors of psychiatry at prestigious medical schools also signed off on such pronouncements by non-profit advocacy associations, and in that manner, share culpability for telling this “falsehood” to the public.
That fraudulent story-telling worked, in the sense of deluding the public. As Moncrieff and colleagues noted, surveys in recent years found that 85% to 90% of the public believed that low serotonin was the cause of depression, and that antidepressants helped fix that imbalance.
There you have the basis for a class action lawsuit: the psychiatric community long ago knew that the low-serotonin story of depression hadn’t panned out, yet the American Psychiatric Association, pharmaceutical companies, and scientific advisory councils told the public otherwise, and this created a societal belief in that false story. The surveys prove that many millions of patients acted upon that falsehood and incorporated it into their sense of self.
The Legal Standard for Medical Fraud
In the wake of World War II, the discovery of Nazi medical experiments on Jewish prisoners and the mentally ill led to the principle, codified in law in the United States, of the duty to provide volunteers in research studies with informed consent. Potential study subjects need to be informed about the risks of a study before they can give consent.
In the 1950s and 1960s, this principle of informed consent was extended to ordinary medical care. The principle is grounded in the concept of personal autonomy: the individual has a right to self-determination. A 1972 landmark case in federal court, Canterbury v. Spence, ruled that providing patients with informed consent was not just an ethical obligation, but a legal one. The court wrote:
“The patient’s right of self-decision shapes the boundaries of the duty to reveal. That right can be exercised only if the patient possesses enough information to enable an intelligent choice.”
The court also set forth a standard for assessing whether this legal obligation had been met: “What would a reasonable patient want to know with respect to the proposed therapy and the dangers that may be inherently or potentially involved?”
While it is the physician or medical caregiver who is required to obtain the informed consent of the patient, this legal standard clearly imposes an ethical duty, by proxy, on the medical specialty that provides individual physicians with the information that should be disclosed. The medical specialty must provide physicians with the best possible accounting of the risks and benefits of any proposed therapy, and in its communications to the public, do the same.
The diagnosis of a disease is obviously a first step in obtaining informed consent. What is the illness that needs to be treated? If the presenting symptoms do not lead to a diagnosis with a known pathology, that is okay—the absence of knowledge helps inform the patient’s decision-making. If it isn’t understood why a drug works, that is okay too. Once again, the absence of knowledge helps inform the patient’s decision-making. At that point, the patient can focus on the risks and benefits of the proposed treatment: what have clinical studies shown?
The chemical imbalance story violated those principles at every step. Patients were informed that they had a known pathology, and that an antidepressant fixed that pathology. That was a story of an antidote to a disease, and thus was a medically necessary treatment. If a patient didn’t take the antidepressant, he or she could expect to continue to suffer from depression.
This isn’t simply a failure to give patients the information needed to make an “informed choice.” Instead, from a legal standpoint, this is a case of a patient being told a lie.
Here is how one Arizona law firm describes the legal consequences for a doctor that lies to a patient:
“You can sue your doctor for lying, provided certain breaches of duty of care occur. A doctor’s duty of care is to be truthful about your diagnosis, treatment options, and prognosis. If a doctor has lied about any of this information, it could be proof of a medical malpractice claim. The law considers it medical negligence if a doctor fails to provide the truth for informed consent, which may also bring a battery lawsuit.”
Medical malpractice is the charge if the action was due to negligence; medical battery requires the action to be intentional. Here is how a Washington D.C. law firm describes medical battery:
“When you visit a doctor and they prescribe a treatment or procedure, an essential element is your consent. You have the right to know what will be done to you, to learn the risk or potential side effects of a procedure, and to be informed of any alternative treatment options available to you . . . Medical battery occurs when the doctor or other medical professional violates your right to decide what kinds of medical treatments you will receive and which you do not wish to receive.”
The FDA, of course, approved the prescribing of antidepressants for depression. And it may be that many individual prescribers who told their patients that antidepressants fixed a chemical imbalance thought that was true. They believed they were providing patients with “informed consent.”
As such, in this instance of the chemical-imbalance story, the medical malpractice and battery can be understood as not necessarily originating in the doctor-patient interaction, but rather in the telling of a false story to the public by the American Psychiatric Association (APA) and pharmaceutical companies that knowingly promoted this falsehood. The academic psychiatrists that served on the scientific advisory boards of non-profit advocacy organizations that peddled this story share in this collective guilt.
The Trail of Fraud
As is well-known, the low-serotonin theory of depression had its roots in findings, dating back to the 1960s, that the first generation of antidepressants, tricyclics and monoamine oxidase inhibitors, both prevented the usual removal of neurotransmitters known as monoamines from the synaptic cleft between neurons. This led researchers in the 1965 to hypothesize that a deficit in monamines could be a cause of depression.
Once this hypothesis was floated, researchers then sought to determine whether if patients with depression actually suffered from a monamine deficiency. It’s a history of one negative finding after another.
As early as 1974, researchers concluded that all such studies up to that time indicated that “the depletion of brain norepinephrine, dopamine, or serotonin is in itself not sufficient to account for the development of the clinical syndrome of depression.” This was the first round of findings, and after that there was speculation that a monoamine deficit might be present in a subset of depressed patients (as opposed to being a pathology common to all such patients.) In 1984, the NIMH conducted a study to investigate that possibility. Once more, the bottom-line findings were negative, which led the NIMH researchers to conclude that “elevations or decrements in the functioning of serotonergic systems per are not likely to be associated with depression.”
At that point, the hypothesis had been around for nearly two decades and found to be wanting. In the research community, there was a sense that the hypothesis had always presented an overly reductive picture of how the brain functioned, and thus it wasn’t a surprise that research had failed to support the hypothesis. Even so, after that 1984 report, investigators continued to study whether depressed patients suffered from low serotonin, with this research quickening after Prozac arrived on the market in 1988. Many different investigative methods were tried, but once again, the results were negative. The hypothesis was officially buried by the American Psychiatric Association in 1999, when it published the third edition of its Textbook of Psychiatry. The authors of a section on mood disorders even pointed out the faulty logic that had led to the chemical imbalance theory of depression in the first place. They wrote:
“The monoamine hypothesis, which was first proposed in 1965, holds that monoamines such as norepinephrine and 5-HT [serotonin] are deficient in depression and that the action of antidepressants depends on increasing the synaptic availability of these monoamines. The monoamine hypothesis was based on observations that that antidepressants block reuptake inhibition of norepinephrine, 5-HT, and/or dopamine. However, inferring neurotransmitter pathophysiology from an observed action of a class of medications on neurotransmitter availability is similar to concluding that because aspirin causes gastrointestinal bleeding, headaches are caused by too much blood and the therapeutic action of aspirin in headaches involves blood loss. Additional experience has not confirmed the monoamine depletion hypothesis.”1
Other experts in the field echoed this point in the next few years. In his 2000 textbook Essential Psychopharmacology, psychiatrist Stephen Stahl wrote that “there is no clear and convincing evidence that monamine deficiency accounts for depression; that is, there is no ‘real’ monoamine deficit.”2
More such confessions appeared in the research literature, and finally, in a 2010 paper, Eric Nestler, famous for his work on the biology of mental disorders, detailed how the many types of inquiries into the low-serotonin theory had all come to the same conclusion:
“After more than a decade of PET studies (positioned aptly to quantitatively measure receptor and transporter numbers and occupancy), monoamine depletion studies (which transiently and experimentally reduce brain monoamine levels), as well as genetic association analyses examining polymorphisms in monoaminergic genes, there is little evidence to implicate true deficits in serotonergic, noradrenergic, or dopaminergic neurotransmission in the pathophysiology of depression. This is not surprising, as there is no a priori reason that the mechanism of action of a treatment is the opposite of disease pathophysiology.”
This is the research history that psychiatrists today, when asked to comment about Moncrieff’s paper, are referring to when they state, “there is nothing new here.” They are right. The theory was abandoned long ago. In a 2011 blog, Ronald Pies, editor of Psychiatric Times, put it this way: “In truth, the ‘chemical imbalance’ notion was always a kind of urban legend—never a theory seriously propounded by well-informed psychiatrists.”
From a legal standpoint, the APA’s publication of the third edition of its Textbook of Psychiatry in 1999 is the pivotal moment in this history. Up until that time, the argument could be made that while the biology of depression remained unknown, one hypothesis was that it was due to low serotonin, and that there were still efforts to see if that might be true. However, after that date, the APA, the pharmaceutical companies, and the academic psychiatrists that populated the scientific advisory councils had an obligation to inform the public that the low-serotonin theory had not panned out. If instead these three groups informed the public that depressed patients suffered from a chemical imbalance that could be fixed by a drug, they were knowingly telling the public a lie, and thus, by informed consent standards, they were abetting medical malpractice and the medical battery of patients.
And it’s easy to document that is exactly what the APA, the pharmaceutical companies, and the scientific advisory boards did.
The APA’s Promotion of the Chemical Imbalance Story
The APA’s promotion of the chemical imbalance theory of mental disorders can be traced back to 1980, when it published the third edition of its Diagnostic and Statistical Manual. That publication is regularly characterized as a transformative moment for American psychiatry, as this was when the APA adopted a “disease” model for diagnosing and treating psychiatric disorders.
There were no scientific findings that spurred this transformation. The scientific impulse that was present arose from the failure of DSM II: the diagnoses in that edition were understood to “lack reliability and validity.” That led a team of researchers at Washington University in St. Louis to advocate that psychiatry should start fresh: it could develop categories for grouping patients with like symptoms, with the hope that subsequent research would “validate” the groupings as real diseases. DSM II would be abandoned, and new categories would be drawn up for research purposes.
However, during the 1970s, APA leaders spoke of how, in the face of various criticisms, psychiatry was fighting for its survival. Its diagnostic manual was understood to lack validity; psychologists and counselors were offering talk therapies that appeared to be as effective as psychoanalysis; One Flew Over the Cuckoo’s Nest depicted staff in mental hospitals as the truly crazy ones; and an “antipsychiatry” movement described psychiatry as an agency of social control.
The criticism that stung the most was that psychiatrists were not “real doctors.” There was an obvious solution that beckoned: if they adopted a disease model, they could present themselves as physicians who treated real diseases. This would enable them to don the “white coat”—both figuratively and literally—that society recognized as the garb of “real” doctors.
DSM III, said APA president Jack Weinberg in 1977, would “clarify to anyone who may be in doubt that we regard psychiatry as a specialty of medicine.”3
Once DSM III was published, the APA set out to market its new disease model to the public. In 1981, it established a “division of publications and marketing” to “deepen the medical identification of psychiatrists.” That same year it established a press to bring “psychiatry’s best talent and current knowledge before the reading public.” It developed a nationwide roster of experts to promote this disease model, and it set up a “public affairs institute” to run workshops that trained its members “in techniques for dealing with radio and television.”4
This PR effort told of a revolution in psychiatry, with the media informed that researchers were discovering the very “molecules” that caused psychiatric symptoms. The APA held “media days” to promote this understanding, with awards given to media that reported on this revolution, and soon newspapers and magazines were writing stories about extraordinary advances that heralded a day when mental disorders could be “cured.”
The Baltimore Sun, in a seven-part series titled “The Mind-Fixers,” which won a Pulitzer Prize for expository journalism in 1984, described the revolution in this way:
“For a decade and more, research psychiatrists have been working quietly in laboratories, dissecting the brains of mice and men and teasing out the chemical formulas that unlock the secrets of the mind. Now, in the 1980s, their work is paying off. They are rapidly identifying the interlocking molecules that produce human thought and emotion . . . As a result, psychiatry today stands on the threshold of becoming an exact science, as precise and quantifiable as molecular genetics. Ahead lies an era of psychic engineering, and the development of specialized drugs and therapies.”5
Pharmaceutical companies, of course, were thrilled with the APA’s adoption of a disease model, for they understood it would greatly expand the market for their drugs, and they began funneling money to the APA and to psychiatrists at academic medical centers to support this PR effort.
The chemical imbalance story served, in essence, as the soundbite that could best sell this disease model to the public. It was a claim that fit into a larger societal narrative about the march of medicine in the 20th century: insulin as a treatment for diabetes, antibiotics for infectious diseases, a vaccine for polio, and so forth. Now it was psychiatry’s turn to take its place at the head of this parade.
The public began hearing this soundbite immediately after DSM III was published. In 1981, an Associated Press article featuring an interview with University of Chicago psychiatrist Herbert Meltzer informed readers that “researchers believe clinical depression is caused by a chemical imbalance in the brain,” and that there were already two drugs in development that “restore the chemical imbalance” to normal.6
Three years later, Nancy Andreasen, who would soon become editor-in-chief of the American Journal of Psychiatry, published a best-selling book titled The Broken Brain: The Biological Revolution. The new understanding in psychiatry, she wrote, was that the “major psychiatric illnesses are diseases,” and that each “different illness has a different specific cause . . . there are many hints that mental illness is due to chemical imbalances in their brain and that treatment involves correcting these chemical imbalances.”7
Eli Lilly brought Prozac to market in 1988, and soon the public was hearing that this “selective serotonin reuptake inhibitor” restored serotonin to normal levels, and thus was like “insulin for diabetes.” New York magazine featured the pill on its cover: “Bye, Bye Blues” declared the headline.8 Newsweek’s did as well, with this headline atop the pill: “Prozac, A Breakthrough Drug for Depression.”9
Magazine and newspaper stories told of how patients were feeling better than ever. In the spring of 1990, the New York Times, in an article by Natalie Angier, who arguably was the nation’s most well-known science writer, informed readers that “all antidepressants work by restoring the balance of neurotransmitter activity in the brain, correcting an abnormal excess or inhibition of the electrochemical signals that control mood, thoughts, appetite, pain, and other sensations.” This new drug, Dr. Francis Mondimore told Angier, “is not like alcohol or Valium. It’s like antibiotics.”
Television shows weighed in with a similar message, and on 60 Minutes, Lesley Stahl told the inspiring story of a woman, Maria Romero, who, after a decade of horrible depression, had been reborn on Prozac. “Somebody, something left my body and another person came in,” Romero said. Stahl explained the biological cure that was at work: “Most doctors believe that chronic depression like Romero’s is caused by a chemical imbalance in the brain. To correct it, the doctors prescribed Prozac.”10
Sales of Prozac soared, and as other drug companies brought new “SSRI” antidepressants to market—Zoloft, Paxil, Celexa, Lexapro, and so forth—they relied on the chemical imbalance soundbite to market their products. The National Alliance on Mental Illness grew in prominence during this period, and its core message was that psychiatric disorders were diseases caused by chemical imbalances in the brain, and that psychiatric drugs fixed those imbalances.

Source: Lacasse JR, & Leo J. (2005). Serotonin and Depression: A Disconnect between the Advertisements and the Scientific Literature. PLoS Med 2(12): e392. https://doi.org/10.1371/journal.pmed.0020392
The American population, and populations around the globe, came to understand this story as scientific truth. The new millennium arrived, and although the APA’s own textbook had declared the low-serotonin theory dead and buried, the APA publicly doubled-down on the chemical imbalance story, informing the public that it was now proven.
“In the last decade, neuroscience and psychiatric research has begun to unleash the brain’s secrets,” wrote APA president Richard Harding, in a 2001 article published in Family Circle. “We now know that mental illnesses—such as depression or schizophrenia—are not ‘moral weaknesses’ or ‘imagined’, but real diseases caused by abnormalities of brain structure and imbalances of chemicals in the brain.”11
In the same issue, future APA president Nada Stotland informed readers that antidepressants “restore brain chemistry to normal.”
And the public believed. On May 4, 2005, the APA issued a press release celebrating the fact that a survey it conducted had found that “75 percent of consumers believe that mental illnesses are usually caused by a chemical imbalance in the brain.” This, said APA president Steven Sharfstein, was evidence of “good news for [public] understanding of mental health.” A psychiatrist, the press release helpfully noted, was “a specialist specifically trained to diagnose and treat chemical imbalances.”
That same year, the APA published its “Let’s Talk Facts about Depression” brochure, which delivered the same message: “Antidepressants may be prescribed to correct imbalances in the levels of chemicals in the brain.”
The APA’s “public education website” continued to tell of chemical imbalances for the next 16 years. Finally, in early 2021, Ronald Pies wrote that he had, at last, managed to get the APA to “delete” that message.
Even so, the APA website still tells the public a limited version of that story. Visitors to a page titled “What is Depression” learn that “brain chemistry may contribute to an individual’s depression and may factor into their treatment. For this reason, antidepressants may be prescribed to modify one’s brain chemistry.”
The Scientific Advisory Councils
In the 1980s and 1990s, the pharmaceutical companies—and the American Psychiatric Association—realized that non-profit advocacy organizations, like the National Alliance on Mental Illness, could help them sell their disease model to the public and inform the public of the effectiveness of psychiatric drugs. Pharmaceutical money flowed to NAMI and other advocacy organizations, and soon the academic psychiatrists that served as industry thought leaders were populating the scientific advisory councils of the non-profit advocacy groups.
In a 2014 blog published on Mad in America, Philip Hickey identified three prominent consumer organizations that informed the public that depression was due to a chemical imbalance, and published the names of the psychiatrists that served on their scientific advisory boards. Here is the list:
Child and Adolescent Bipolar Foundation
-
- Joseph Biederman, MD, Professor of Psychiatry at Harvard Medical School
- Gabrielle Carlson, MD, Professor of Psychiatry and Pediatrics, Director of Child and Adolescent Psychiatry, Stonybrook State University
- Kiki Chang, MD, Associate Professor and Director of Pediatric Bipolar Disorders Program, Child and Adolescent Psychiatry, Stanford University
- Melissa DelBello, MD, Professor of Psychiatry and Pediatrics, University of Cincinnati
- Robert L. Findling, MD, Professor of Child & Adolescent Psychiatry, Case Western Reserve University
- Janet Wozniak, MD, Assistant Professor of Psychiatry, Harvard Medical School
Depression and Bipolar Support Alliance
-
- Gregory E. Simon, MD, MPH, Psychiatrist and Senior Investigator, GroupHealth Research Institute, Seattle
- Michael E. Thase, MD, Professor of Psychiatry. University of Pittsburgh
- Mark S. Bauer, MD, Associate Professor of Psychiatry, Brown University School of Medicine
- Joseph R. Calabrese, MD, Professor of Psychiatry and Director of Mood Disorders Program, Case Western Reserve University
- David J. Kupfer, MD, Professor & Chairman Department of Psychiatry, University of Pittsburgh
- George S. Alexopoulos, MD, Professor of Psychiatry, Cornell University
- Gary Sachs, MD, Director, Bipolar Research Program, Harvard University
- Mark A. Frye, MD, Professor of Psychiatry, Mayo Clinic
- J. Raymond DePaulo Jr. MD, Professor of Psychiatry, Johns Hopkins
- William Beardslee, MD, Psychiatrist-in-Chief, Children’s Hospital, Boston
NAMI
-
- Nancy Andreasen, MD, Chair of Psychiatry at The University of Iowa College of Medicine.
- Ellen Frank, PhD, Professor of Psychiatry and Psychology at the University of Pittsburgh School of Medicine.
- David Kupfer, MD, Professor of Psychiatry and Professor of Neuroscience, University of Pittsburgh School of Medicine.
- Jeffrey Lieberman, MD, Chair of Psychiatry, Columbia University and Director of the New York State Psychiatric Institute.
- Henry Nasrallah, MD: Associate Dean; Professor of Psychiatry and Neuroscience, University of Cincinnati.
- Charles Nemeroff, MD: Chair of Psychiatry and Behavioral Sciences, University of Miami Health System.
- S. Charles Schulz, MD: Professor and Chair, Department of Psychiatry, University of Minnesota Medical School.
The names on the list constituted a “who’s who” of prominent academic psychiatrists at that time, many of whom were known to have been paid hundreds of thousands of dollars for their “thought leader” services to industry. Theirs was a collective voice informing the American public that depression was due to a chemical imbalance, which could be successfully treated by antidepressants that helped correct that imbalance. Fifteen years after the APA declared the low-serotonin theory dead, antidepressants—on these websites—were still being presented as an antidote to a disease.
While many consumer organizations have now scrubbed such claims from their sites, they have not disappeared altogether. For example, the Child Mind Institute website, on a page titled “Medication for Kids with Depression,” provides this description of antidepressants:

URL: https://childmind.org/article/medication-for-kids-with-depression/
The founder of the Child Mind Institute is one of the most prominent child psychiatrists in the United States, Harold Koplewicz. He is chair of the Department of Child and Adolescent Psychiatry at NYU School of Medicine and has been editor-in-chief of the Journal of Child and Adolescent Psychopharmacology since 1997. A primary mission of the Child Mind institute, his profile page states, is to “educate and empower parents by providing trustworthy information and resources.”
Pharmaceutical Companies
As anyone who watched television in the first decade of the new millennium knows, pharmaceutical companies used the chemical imbalance story to sell their antidepressants. Pfizer, for instance, flooded the airways with its “Sad Blob” ad, and if you pay close attention, you’ll see that Pfizer knows the chemical imbalance story is unfounded. Yet, it uses the chemical story to sell its drug anyway. It accomplishes this verbal sleight of hand in two brief sentences: “While the cause (of depression) is unknown, depression may be related to an imbalance of natural brain chemicals between nerve cells in the brain. Prescription Zoloft works to correct this imbalance.”
The ad closes with this reminder: “When you know more about what is wrong, you can help make it right.”
Such is the trail of fraud that lawyers could present if they mounted a class action lawsuit. They could detail how there is a long line of research, dating back to the 1970s, that failed to find that low serotonin was a cause of depression. They could show that in 1999 the APA’s own textbook declared the theory dead and buried. And then they could detail how the APA, scientific advisory boards at advocacy organizations, and pharmaceutical companies continued to promote the chemical imbalance theory after that, with antidepressants presented as drugs that fixed chemical imbalances. That continued promotion is evidence that from 1999 forward these three groups were knowingly promoting a falsehood, which patients could be expected to act upon.
This is evidence of medical fraud—and, one might say, societal medical battery—committed on a grand scale.
The Other Half of the Chemical Imbalance Story
While researchers did not discover that people diagnosed with depression had abnormal serotonin systems before they took an antidepressant, they did discover that the compounds induced the very abnormality hypothesized to cause the disorder in the first place.
The basic mechanism of an SSRI is well known. When a presynaptic neuron releases serotonin into the tiny gap between neurons (known as the synapse), the serotonin molecules bind with receptors on the post-synaptic neuron, and then, in a flash, the serotonin is removed from the synapse. An enzyme metabolizes a small amount of the serotonin; the rest is quickly pumped back into the presynaptic neuron, entering via a channel known as SERT. In a 1975 paper, Eli Lilly scientists reported that fluoxetine, the compound that would be marketed as Prozac, blocked this reuptake process, causing a “pile-up of serotonin at the synapse.”
However, the presynaptic neuron has “autoreceptors” on its terminal membrane that monitor serotonin levels in the synapse, and with serotonin levels piling up, these autoreceptors begin to scream, as one scientist quipped, “turn off the serotonin machine.” The presynaptic neurons begin to fire at a lower rate, while the postsynaptic neurons decrease the density of their receptors for serotonin.
In other words, the drug puts down the accelerator on serotonergic transmission, and the brain responds by putting on the brake.
Over time, other changes may kick in. There are feedback loops that connect different neurotransmitter systems to each other, and so this initial response to the drug is likely a prelude to a host of downstream changes that have yet to be identified. However, the initial response to fluoxetine was fleshed out early, and it told of how fluoxetine, rather than normalize serotonergic pathways, induced profound abnormalities in this system.
In 1996, NIMH director Steven Hyman published a paper titled “Initiation and Adaptation: A Paradigm for Understanding Psychotropic Drug Action” that told of how all psychiatric drugs could be understood to create abnormalities in brain function.
Psychiatric drugs, he wrote, create “create perturbations in neurotransmitter function.” In response to this perturbation, the brain goes through a series of compensatory adaptations, and in each instance, the immediate adaption is for the brain to oppose the effects of the drug. An antipsychotic blocks dopamine transmission, and in response the brain’s dopaminergic pathways spring into high gear, at least for a time. An antidepressant ups serotonergic levels in the synapse, and in response, the brain puts a brake on its serotonergic pathways. These compensatory adaptations, Hyman wrote, “are rooted in homeostatic mechanisms that exist, presumably, to permit cells to maintain their equilibrium in the face of alterations in the environment or changes in the internal mileu.”
Hyman was describing adaptive changes known as “oppositional tolerance” to a drug. After a period of time, he continued, the “chronic administration” of the drug causes “substantial and long-lasting alterations in neural function.” As part of this process, there are changes in intracellular signaling pathways and gene expression. After a few weeks, he concluded, the person’s brain is functioning in a manner that is “qualitatively as well as quantitatively different from the normal state.”
“Qualitatively as well as quantitatively different” than normal. Indeed, two Eli Lilly scientists, Ray Fuller and David Wong, early on observed that fluoxetine, since it disrupted serotonergic pathways, could be used to study “the role of serotonin neurons in various brain functions—behavior, sleep, regulation of pituitary hormone releases, pain responsiveness and so on.” To conduct such experiments, researchers could administer fluoxetine to animals to observe which functions became compromised. They would look for pathologies to appear.
Such was the state of scientific knowledge about antidepressants as a treatment for depression by the end of the 1990s. There was no evidence that depressed patients suffered from low serotonin before they took an antidepressant, but research had shown that once they did, their brain would begin functioning in a manner that was “qualitatively as well as quantitatively different from the normal state.”
Antidepressants were promoted to the public as “normalizing agents,” when in fact researchers knew they were “abnormalizing” agents.
Harm Done
In their responses to Moncrieff’s paper, many psychiatrists sounded a “no harm, no foul” argument. “Antidepressants work,” they stated, and thus the prescribing of antidepressants was a helpful practice, even if there was some confusion about the cause of depression and what the drugs did.
Here is how Massachusetts psychiatrist Daniel Carlat put it, in his interview on National Public Radio’s “On Point” program:
“Doctors don’t know exactly about how (antidepressants) work. Patients do want to know there is an explanation out there. And there are times when we do have to give them a shorthand explanation, even if it is not entirely accurate.”
In terms of harm done by the chemical imbalance lie, whether an antidepressant reduces the patient’s symptoms over some period of time is beside the point. The chemical imbalance story informs the patient that he or she suffers from a brain pathology, which requires treatment with a drug that treats that pathology. That is a diagnostic story that changes a patient’s sense of self and understanding of his or her own mind. Moreover, the treatment is designed to change how the individual emotionally responds to the world—this is an intervention of a most profound sort.
Indeed, the decision to take an antidepressant puts the patient on a different life course. It puts a person on a path of a medicated future, as opposed to the life the person had known before and the life that the person might have if he or she sought some other non-medical form of treatment. In that sense, the decision of whether to take an antidepressant acts as the proverbial fork in the road—two different lives stretch ahead.
That is the harm done when the chemical imbalance story was told to patients seeking help for depression: They made a profound decision about their future based on a lie.
The chemical imbalance story also did harm at a societal level. It remade our collective sense of self.
Before Prozac arrived on the market, a NIMH survey found that only 12% of American adults said they would take a pill to treat depression. This was a survey that told of a public that understood, at some level, that to experience periods of suffering was normal, that life had its ups and downs, and that often people could call on an inner resilience—and environmental support—to lead them out of the tunnel of darkness.
But then came the selling of psychiatry’s disease model, and in fairly quick order the public came to see human nature in a new light: our moods were directed by a molecule called serotonin, and if a person experienced depression, they had, in the words of Nancy Andreasen, a “broken brain.”
This is a conception that also stifles political efforts to create a society that better nurtures mental and emotional well-being. The chemical imbalance story placed the cause of depression within the brain of the individual, which fits a neoliberal agenda, but produces a blindness to social conditions that promote suffering and depression: poverty, lack of access to decent housing, poor childcare support, and so on.
As Moncrieff wrote, surveys have found that more than 85% of the public came to believe that depression is caused by low serotonin. That number tells of a conspiracy—by a guild, pharmaceutical companies, and academic psychiatrists—that profoundly betrayed our society. They told us a story that their own research had shown to be false, and they did so because it benefitted guild interests and the financial interests of pharmaceutical companies. As for the members of the scientific councils, they were signing off on a story that kept them in good standing as industry “thought leaders” and further burnished their public reputations as leaders in the field.
From a legal standpoint, it doesn’t really matter whether “antidepressants work.” Lying to patients and to society is a form of medical battery, and any possible therapeutic benefit doesn’t excuse that deception. However, when that “antidepressants work” claim is examined, it can be seen that it is a continuation of the false marketing of these drugs.
Do Antidepressants Work?
When the public is told that a drug “works,” they are being led to believe that most people who take the drug can expect to receive a benefit. An antibiotic, for instance, is a drug that can be said to “work.” When penicillin and other antibiotics were introduced in the 1940s, they cured bacterial infections and any number of bacterial illnesses: pneumonia, scarlet fever, diphtheria, and tuberculosis, to name a few. But an antidepressant cannot be said to work in this way.
What can be said is that there are clinical studies that provide information about the possible risks and benefits of antidepressants, both over the short-term and long-term. The relevant information can be grouped into three types.
Placebo-controlled trials
When psychiatrists state that antidepressants “work,” they are mostly citing findings from industry-funded trials of the drugs. Meta-analyses of these short-term trials have found that the difference in the reduction of symptoms between the drug-treated and placebo groups is about two points on the 52-point Hamilton Depression Rating Scale. While this difference may be statistically significant, it is of questionable clinical significance.
The best way to understand this difference is to look at its effect size. At an individual level, responses fall along a bell curve, and a visualization of the effect size reveals how the bell curves for the placebo and drug-treated groups differ. Researchers have concluded that the “effect size” in the industry trials is 0.3 (effect sizes can range from 0 to 3.0).
As the graphic below reveals, when a treatment has an effect size of 0.3, there is an 88% overlap in the bell curves of the two groups. That means you need to treat eight people with an antidepressant to produce one additional person who benefits from the treatment. Seven of eight treated with the drug will be exposed to the adverse effects of the drug without any additional benefit beyond placebo.
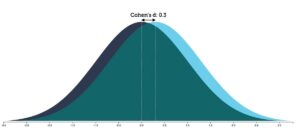
Graphic by Kristoffer Magnusson, http://rpsychologist.com/de/cohend/
Studies in “real-world” patients
The industry-funded trials are typically conducted in a subset of patients who could be expected most likely to respond well to the drug (without comorbidities and so forth), and thus are understood to not necessarily reflect outcomes in the general population. Studies in “real-world” patients, which in the U.S. are usually funded by the National Institute of Mental Health, are typically not placebo-controlled, but rather simply seek to assess what percentage of patients respond, in a significant way, to the treatment.
These studies have reported lower “response” rates to antidepressants than the industry-funded trials do, and particularly poor stay-well rates.
In a 2004 study of 118 real-world patients treated with an antidepressant, only 26% of the patients “responded” to the treatment (meaning that their symptoms decreased at least 50% on a rating scale), and fewer than 13% were in remission at the end of 12 months. These findings, the investigators concluded, “reveal remarkably low response and remission rates.”
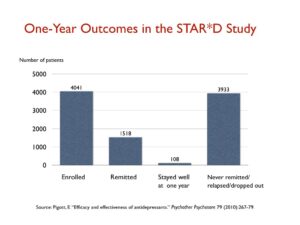
The STAR*D trial funded by the NIMH, which was heralded as the largest antidepressant trial ever conducted, produced similarly poor results in “real-world” patients. The 4,041 patients enrolled into the trial were given up to four trials on an antidepressant to find one that led to remission (defined as a score below 7 on the Hamilton scale), and only 38% achieved this level of improvement.
Those who remitted were then whisked into a longer-term follow-up study, where they would get the best clinical care possible. Yet, at the end of one year, only 108 of the 4,041 patients entered into the STAR*D trial had remitted, and then stayed well and in the trial to its end. All the rest had either never remitted, relapsed, or dropped out at some point. That is a one-year stay-well rate of 3%.
More recently, an international group of researchers, in a study of 1,217 patients diagnosed with major depressive disorder, reported that only 24% responded to treatment with an antidepressant (either alone or in combination with psychotherapy.) Thirty-four percent were “non-responders” to antidepressants, and the remaining 41% fared so poorly that they were deemed “treatment resistant.” Nearly 60% ended up ended up on multiple medications, including multiple antidepressants, antipsychotics, benzodiazepines, and other combinations of drugs.
These real-world studies were not placebo controlled, and thus there wasn’t a comparison with a similar group of untreated patients. This raises the obvious question: What is the natural course of depression?
Prior to the antidepressant era, depression was understood to be an episodic disorder, as opposed to a chronic illness. Spontaneous recovery rates were said to exceed 50% within a few months, with this recovery rate reaching 85% or so by the end of one year. As Dean Schuyler, head of the depression section at the NIMH explained in a 1974 book, most depressive episodes “run their course and terminate with virtually complete recovery without specific intervention.”12
However, after the introduction of antidepressants, the disorder began running a much more chronic course. In the 1970s, several clinicians observed that the drug was causing a “chronification” of the disease, and subsequent epidemiological studies confirmed that the long-term course of depression had changed. The APA’s 1999 textbook summed up the findings from the newer studies: “Only 15% of people with unipolar depression experience a single bout of the illness,” and for the remaining 85%, with each new episode, remission becomes “less complete and new recurrences develop with less provocation.”13
With the outcomes for medicated patients so poor, the NIMH funded a study to assess the course of “untreated depression” in the modern era. Perhaps the natural course of depression had changed? In 2006, researchers reported that 23% of the non-medicated patients recovered in one month; 67% in six months; and 85% within a year. These were outcomes, the researchers concluded, that echoed those in the pre-antidepressant era. “If as many as 85% of depressed individuals who go without somatic treatment spontaneously recover within one year, it would be extremely difficult for any intervention to demonstrate a superior result to this,” they wrote.

Such is the risk-benefit equation that emerges from studies of real-world patients. Perhaps 25% will respond to an antidepressant, and perhaps 15% or so respond to treatment and stay well. There is also reason to believe that the one-year recovery rate for untreated patients is much higher than that.
Long-term outcomes
The industry-funded trials provide a risk-benefit equation at the end of six weeks on the drug. The clinical studies in real-world patients provide information about the percentage of people diagnosed with major depression who, at some point during studies of longer duration (typically six months to a year), will respond to an antidepressant and still be well at the end of the study. The third question that needs to be assessed is this: How do patients treated with antidepressants fare over longer periods of time—two years or more?
This question harkens back to the same one that arises in the clinical studies of one year in length: what is the natural long-term course of the illness? For an antidepressant to be effective over the long term, it would need to improve on that natural recovery rate.
Unfortunately, there is abundant evidence that antidepressants, on the whole, increase the risk that a person will become chronically depressed and functionally impaired. I reviewed that collection of evidence in Anatomy of An Epidemic; a summary of that research can be found here on Mad in America.
In the mid 1990s, Italian psychiatrist Giovanni Fava raised this concern in a series of papers. He wrote:
“Antidepressant drugs in depression might be beneficial in the short term, but worsen the progression of the disease in the long term, by increasing the biochemical vulnerability to depression . . . Use of antidepressant drugs may propel the illness to a more malignant and treatment unresponsive course.”
In his articles on this topic, Fava noted that antidepressants induce changes in the serotonin system the opposite of their intended effect, and reasoned that this might be the mechanism that “sensitized” the brain to depression.
In 2012, American psychiatrist Rif El-Mallakh, an expert in mood disorders, concluded that SSRIs could induce a chronic “tardive dysphoria.” He noted that up to 40% of patients initially treated with an antidepressant end up “treatment resistant,” and up to 80% maintained on the drugs suffer a recurrence of symptoms.
“A chronic and treatment-resistant depressive state is proposed to occur in individuals who are exposed to potent antagonists of serotonin reuptake pumps (SSRIs) for prolonged periods. Due to the delay in the onset of this chronic depressive state, it is labeled tardive dysphoria. Tardive dysphoria manifests as a chronic dysphoric state that is initially transiently relieved by—but ultimately becomes unresponsive to—antidepressant medication. Serotonergic antidepressants may be of particular importance in the development of tardive dysphoria.”
Such is the gap between what depressed patients—and society at large—have been told about antidepressants for the past 30-plus years and the story told in the scientific literature. The public was led to believe that antidepressants fixed a chemical imbalance in the brain and thus could be considered an antidote to the pathology that caused depression, and that clinical studies have shown that these drugs “work.” In fact, the research literature told the following story:
- Depression is not caused by a known chemical imbalance in the brain
- An antidepressant causes the brain to begin functioning in a manner that is both “qualitatively and quantitatively different” than normal
- In industry-funded trials, only one in eight patients could be said to benefit from the treatment
- Studies in real-world patients found that only a minority of patients respond to an antidepressant and relatively few remain well at the end of one year
- Long-term outcomes for treated patients are particularly poor, and there is evidence that their use increases the risk that a person will become chronically ill
This, of course, is information that would enable patients to make an informed choice about whether to take an antidepressant. Yet—and this is an example of how the APA continues to misinform the public—here is what the APA currently tells the public about the efficacy of antidepressants:
“Between 80% and 90% of people with depression eventually respond well to treatment. Almost all patients gain some relief from their symptoms.”
Why a Lawsuit Is Needed
All societies need their medical communities to provide the public with honest information about what is known about the nature of an illness, and the risks and benefits of a treatment for that illness.
The chemical imbalance story of depression violated that obligation of honesty, and egregiously so. In lieu of information necessary for a depressed patient to give informed consent, patients—and the public—were told a false story that benefitted guild interests and the financial interests of pharmaceutical companies. In essence, a marketing story was substituted for a scientific one.
Mad in America has published numerous stories by people who were told they suffered a chemical imbalance in the brain, and whose lives then crashed and burned after they took an antidepressant, with so many ending up on drug cocktails. Their stories just begin to hint at the extraordinary harm done by the chemical-imbalance deception.
And yet, even as psychiatrists have said there was “nothing new” with Moncrieff’s paper, there has been no public admission of wrongdoing, or apology, for the misleading of patients and society in this way for decades. Carlat, in his comments on NPR’s On Point show, even justified it, at least to a degree, putting it into the category of a little white lie. At times, he said, psychiatric patients need to be given information about psychiatric drugs that “is not entirely accurate.”
Meanwhile, the APA just keeps on with its propaganda, telling the public that nearly all patients eventually respond well to antidepressants. That is a whopper that trumps the antidepressants-fix-chemical-imbalance story for its mendaciousness.
This is why a class-action lawsuit is needed. To this point, those promoting the chemical imbalance suffered have suffered no cost for doing so. Rather, money has been made, careers have been burnished, and all the while our society has borne the cost.
A class-action suit would serve society well. It would put teeth into the legal obligation for doctors to provide “informed consent,” and for a medical discipline to provide society with information that met this standard too.
Sources: Much of the history and research recounted here is taken from Anatomy of an Epidemic: Magic Bullets, Psychiatric Drugs and the Astonishing Rise of Mental Illness in America, a book I published in 2010, and Psychiatry Under the Influence: Institutional Corruption, Social Injury, and Prescriptions for Reform, a book I co-wrote with Lisa Cosgrove, published in 2015.
Correction: In the quote above by Ronald Pies, I identified Psychiatric Times as a publication of the American Psychiatric Association. That was in error. Psychiatric Times is a trade publication published by MHS associates.


